
Electrochemical Nanomaterials
The Milliron Group adapts common electrochemical techniques such as cyclic voltammetry, electrochemical impedance spectroscopy and half-cell electrochemical measurements to nanomaterials and extends them with capabilities such as in situ spectroscopic measurements. We utilize our expertise in nanocrystal synthesis to study how changes in size, shape, and doping influence electrochemical properties.
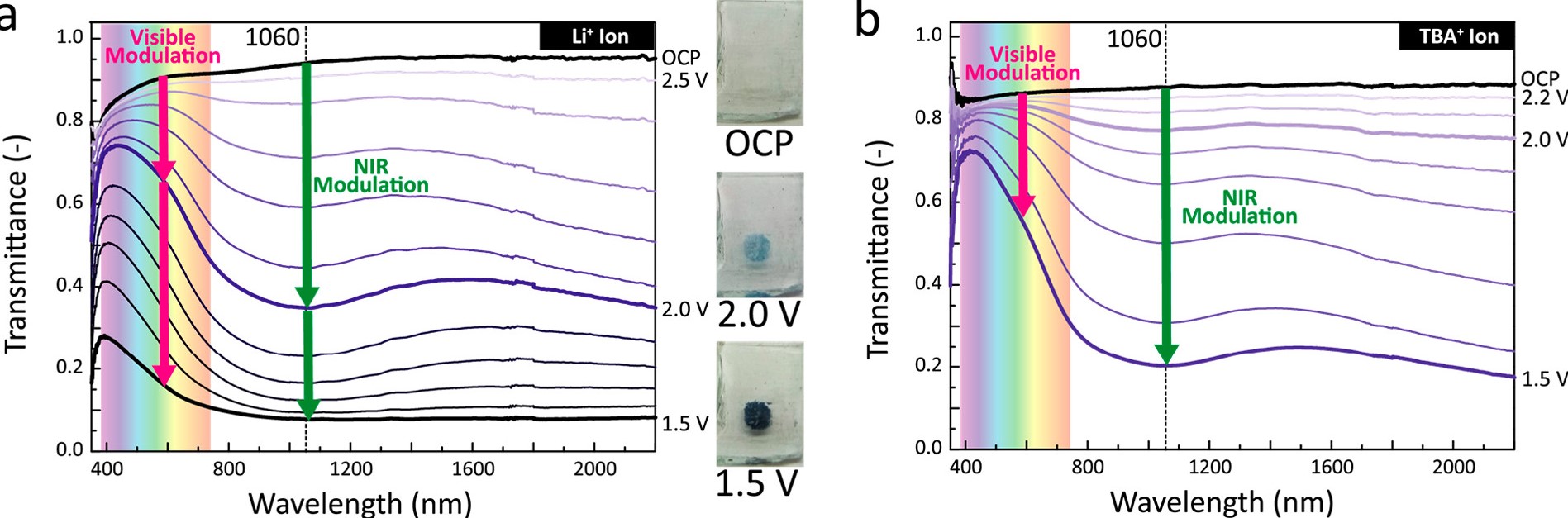
Spectroelectrochemical characterization of ligand-stripped niobium oxide nanoplatelet films. Transmittance spectra of films in (a) 1 M lithium bis(trifluoromethanesulfonyl)imide/tetraethylene glycol dimethyl ether (Li-TFSI/TG) and (b) 0.1 M tetrabutylammonium bis(trifluoromethanesulfonyl)imide (TBA-TFSI)/TG reduced at various potentials (vs Li/Li+) for 5 min by chronoamperometry.[1]
One of our research thrusts in electrochemical materials is studying the electrochromic properties of nanocrystal thin films. We study how the nanostructuring of metal oxides and different electrochemical processes, such as ion intercalation or capacitive charging, influence the electrochromic properties.[2-5] We specialize in developing nanomaterials that allow for selective control of the visible and near-infrared regions of the electromagnetic spectrum, which has potential applications in electrochromic window coatings that can regulate solar lighting and heating. [1] Our experimental set up allows us to simultaneously monitor the UV-Vis-NIR transmittance of thin film samples as a function of electrochemical reduction or oxidation, providing a platform to test and study the electrochromism of materials such as tungsten oxide and niobium oxide. [6,7]
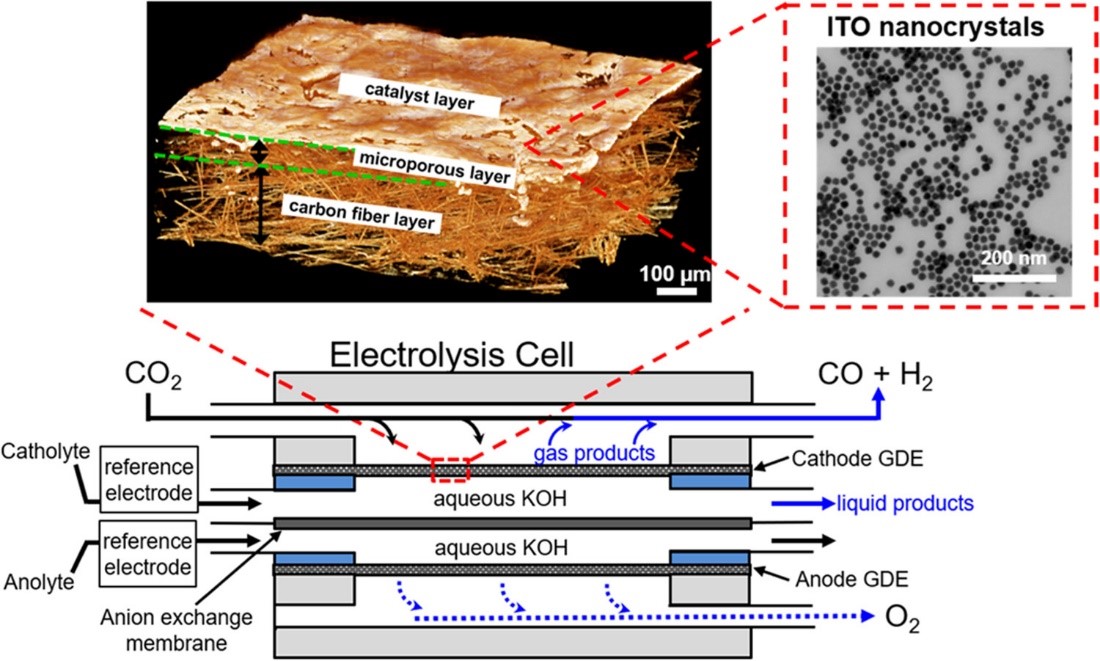
Schematic representation of the microfluidic electrolysis cell (bottom) used in this study for electroreduction of CO2 to CO and formate. Top-right: STEM of the ITO NC catalyst. Top-left: Reconstructed 3D view (obtained from MicroCT data) of a gas diffusion electrode coated with a 5 atom % ligand-stripped ITO catalyst layer (1.0 mg ITO/cm2), deposited via automated airbrushing. [8]
Another research thrust in electrochemical materials is studying how nanocrystal defect chemistry can be used for electrocatalysis. By changing the dopant concentration in tin-doped indium oxide nanocrystals, we can influence activity and product selectivity for electrochemical CO2 reduction. [8] We synthetically tune metal oxide defect chemistry as a handle to further our understanding of reaction mechanisms for sustainable energy research.
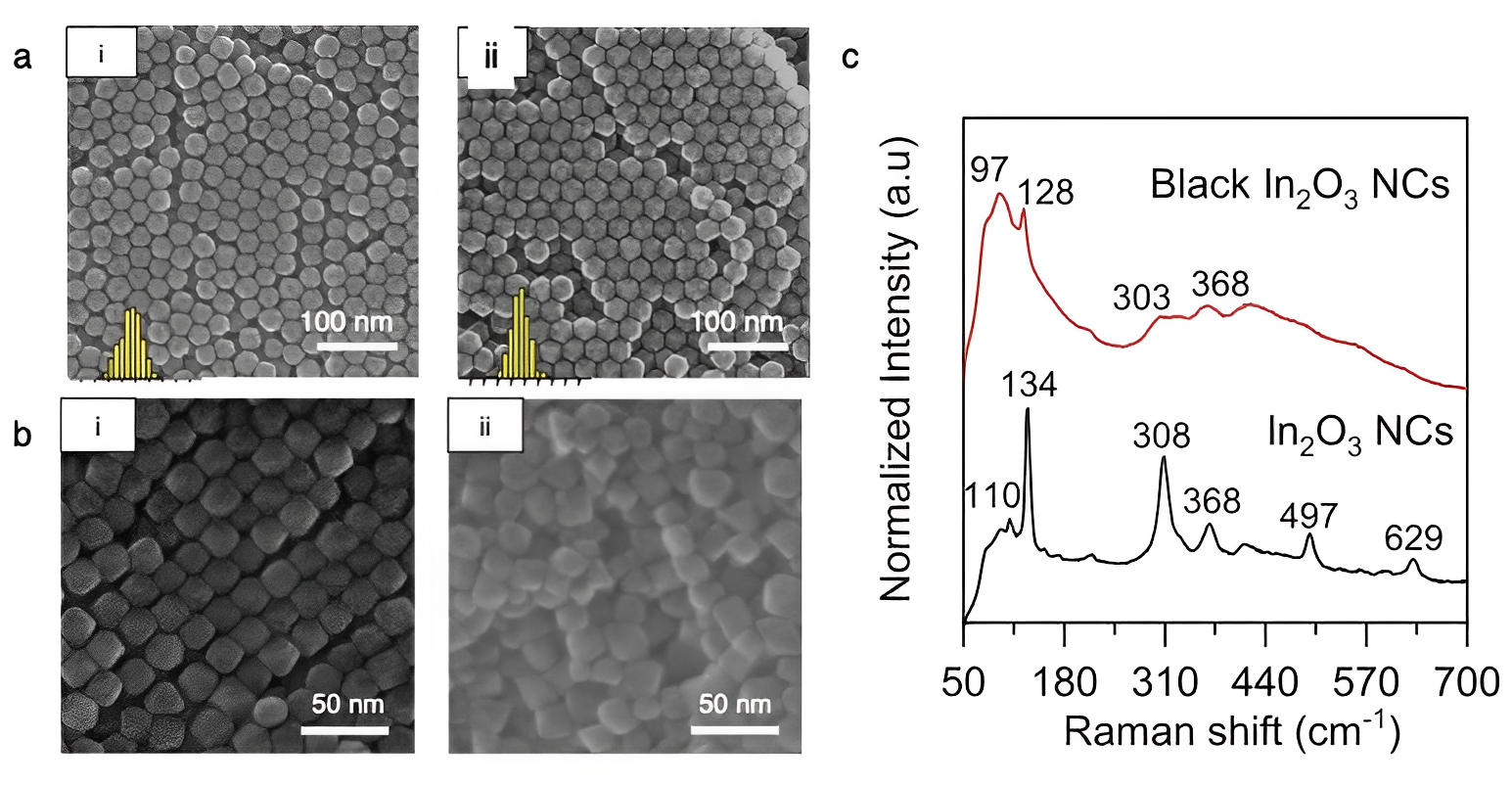
Controlled introduction of vacancies during synthesis of metal oxide nanocrystals. Scanning electron microscopy of stoichiometric (i) and oxygen deficient (ii) nanocrystals with similar morphology, (a) indium oxide and (b) iron oxide. (c) Raman peaks shift due to high oxygen vacancy concentration in black indium oxide nanocrystals. [9]
Lastly, another area of research is focused towards making hydrogen fuel production more viable, both environmentally and economically. For this, we seek to understand the mechanism of electrolytic water splitting reaction over various catalysts and especially metal oxide nanocrystals. With our expertise in synthetic control over compositionally complex metal oxide nanocrystals and their integration in electrochemical systems, we aim to discover principles relating the crystallographic defect chemistry of transition metal oxides to their electrocatalytic activity.
Related papers:
[1] HC Lu, S Ghosh, N Katyal, VS Lakhanpal, IR Gearba-Dolocan, G Henkelman, DJ Milliron. “Synthesis and Dual-Mode Electrochromism of Anisotropic Monoclinic Nb12O29 Colloidal Nanoplatelets,” ACS Nano, 14 (2020), 10068-10082 [link]
[2] HC Lu, BZ Zydlewski, B Tandon, SA Shubert-Zuleta, DJ Milliron. “Understanding the Role of Charge Storage Mechanisms in the Electrochromic Switching Kinetics of Metal Oxide Nanocrystals,” Chem. Mater., 34 (2022), 5621-5633 [link]
[3] S Heo, SH Cho, CJ Dahlman, A Agrawal, DJ Milliron. “Influence of Crystalline and Shape Anisotropy on Electrochromic Modulation in Doped Semiconductor Nanocrystals,” ACS Energy Lett., 5 (2020), 2662-2670 [link]
[4] S Heo, CJ Dahlman, CM Staller, T Jiang, A Dolocan, BA Korgel, DJ Milliron. “Enhanced Coloration Efficiency of Electrochromic Tungsten Oxide Nanorods by Site Selective Occupation of Sodium Ions,” Nano Lett,, 20 (2020), 2072-2079 [link]
[5] CJ Dahlman, S Heo, Y Zhang, LC Reimnitz,D He, M Tang, DJ Milliron. “Dynamics of Lithium Insertion in Electrochromic Titanium Dioxide Nanocrystal Ensembles,” J. Am. Chem. Soc., 143 (2021), 8278-8294 [link]
[6] HC Lu, N Katyal, G Henkelman, DJ Milliron. “Controlling the Shape Anisotropy of Monoclinic Nb12O29 Nanocrystals Enables Tunable Electrochromic Spectral Range,” J. Am. Chem. Soc., 143 (2021), 15745-15755 [link]
[7] BZ Zydlewski, HC Lu, H Celio, DJ Milliron. “Site-Selective Ion Intercalation Controls Spectral Response in Electrochromic Hexagonal Tungsten Oxide Nanocrystals,” J. Phys. Chem. C, 126 (2022), 14537-14546 [link]
[8] HR Jhong, UO Nwabara, SA Shubert-Zuleta, NS Grundish, B Tandon, LC Reimnitz, CM Staller, GK Ong, CA Saez-Cabeza, JB Goodenough, PJA Kenis, DJ Milliron. “Efficient Aqueous Electroreduction of CO2 to Formate at Low Overpotential on Indium Tin Oxide Nanocrystals,” Chem. Mater., 33 (2021), 7675-7685 [link]
[9] K Kim, J Yu, J Noh, LC Reimnitz, M Chang, DR Gamelin, BA Korgel, GS Hwang, DJ Milliron. “Synthetic Control of Intrinsic Defect Formation in Metal Oxide Nanocrystals Using Dissociated Spectator Metal Salts,” J. Am. Chem. Soc., 144 (2022), 22941-22949 [link]