
Phase Stability and Transitions
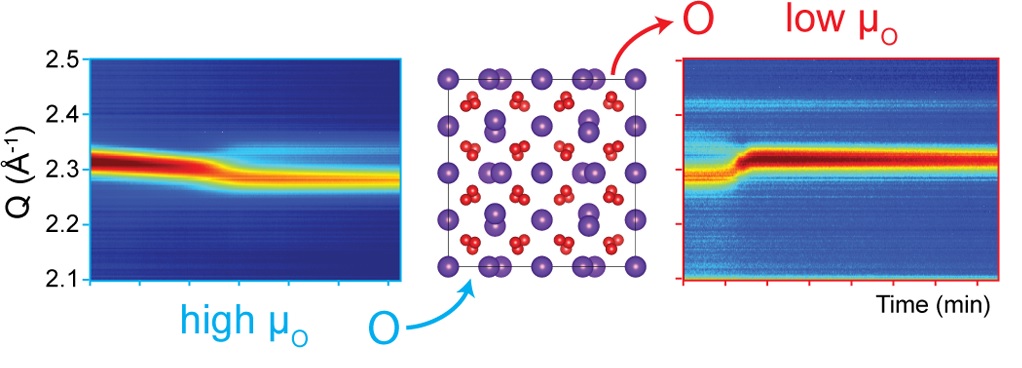
In situ X-ray diffraction of vanadium oxide nanoparticles performed at the National Synchrotron Light Source at Brookhaven National Laboratory. This powerful technique enables our team to study the effects of nanoscale size on phase stability under different annealing conditions [2].
It is well known that the properties of nanomaterials often differ greatly from the bulk properties of the same material composition. In the case of nanocrystals, the formation of metastable phases is possible due to the increased influence of surface energy and surface stress at these size scales. This affords the opportunity to utilize unique properties of these metastable states and manipulate potentially reversible transitions to other phases.
In one example, the Milliron group demonstrated the colloidal synthesis of bixbyite V2O3 [1]. This phase of vanadium oxide has only recently been reported in the literature, and this report is one of the first colloidal syntheses of any vanadium oxide; a class of materials with a wide variety of potential applications, including oxygen storage, catalysis, and thermochromic behavior. To understand these systems we utilize a variety of in situ X-ray and spectroscopic techniques. We reported the reversible incorporation of oxygen in this material, which can be controlled by varying temperature and oxygen partial pressure [2].
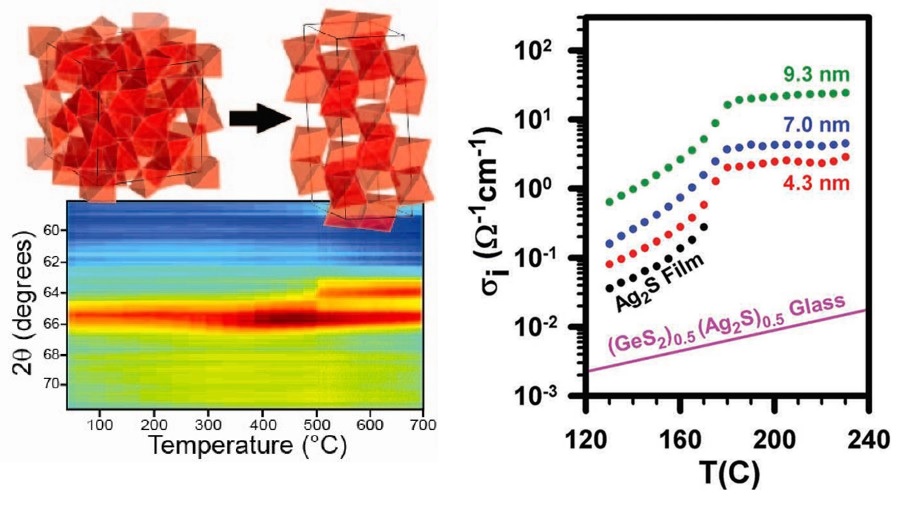
The Milliron group developed a colloidal route to vanadium sesquioxide (V2O3) nanocrystals with a metastable bixbyite crystal structure that is able to undergo thermal phase transformation. [1]. Interface-enhanced ionic conductivity for Ag2S nanocrystal – GeS2 matrix composite materials is observed by controlling the nanocrystal size within the GeS2 matrix [4].
In other examples, we have investigated how nanoparticle size can influence the phase change properties in Cu2ZnSnS4 (CZTS) [3], Ag2S2 [4], and GeTe [5]. These materials can be utilized in a variety of applications (e.g. phase change or resistive switching memory) due to the drastic difference in ionic, thermal, and electrical conductivity observed between the different thermally induced phases. By controlling these properties via colloidal synthesis, we can tailor these systems for particular behaviors.
Related papers:
[1] A Bergerud, R Buonsanti, JL Jordan-Sweet, DJ Milliron. “Synthesis and Phase Stability of Metastable Bixbyite V2O3 Colloidal Nanocrystals,” Chem. Mater. 25 (2013), 3172 - 3179 [link]
[2] A Bergerud, SM Selbach, and DJ Milliron. “Oxygen Incorporation and Release in Metastable Bixbyite V2O3 Nanocrystals,” ACS Nano., 10 (2016), 6147–6155 [link]
[3] A Singh, L Lutz, GK Ong, K Bustillo, S Raoux, JL Jordan-Sweet, and DJ Milliron , “Controlling Morphology in Polycrystalline Films by Nucleation and Growth from Metastable Nanocrystals,” Nano Lett., (2018), Article ASAP, 10.1021/acs.nanolett.8b01916 [link]
[4] RY Wang, R Tangirala, S Raoux, JL Jordan-Sweet, DJ Milliron. “Ionic and Electronic Transport in Ag2S Nanocrystal - GeS2 Matrix Composites with Size-Controlled Ag2S Nanocrystals,” Adv. Mater., 24 (2012), 99-103 [link]
[5] MA Caldwell, S Raoux, RY Wang, HSP Wong, DJ Milliron, “Synthesis and Size-Dependent Crystallization of Colloidal Germanium Telluride Nanoparticles,” J. Mater. Chem., 20, (2010), 1285 [link]