
Interface-mediated Charge Transport
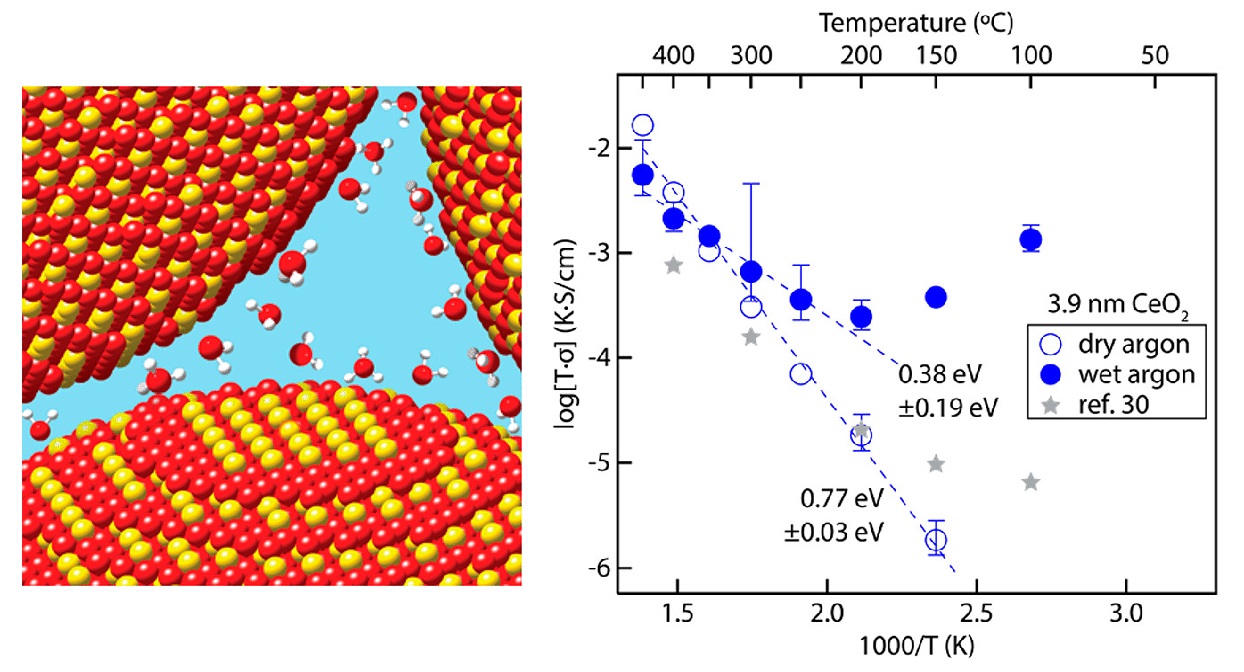
Proton conductivity Arrhenius plots of porous CeO2 nanocrystal film under dry and wet Ar. Proton conduction can occur by hopping along the oxide surface or by exchange with adsorbed water at the nanocrystal surface. [1]
In nanoscale systems, the density of interfaces becomes very large and can strongly influence or even dominate the charge transport properties of nanomaterials. The Milliron group studies charge transport in nanocrystal films and composites, seeking to understand how factors such as nanocrystal size/surface area, doping, polymer or inorganic glassy matrices, and processing techniques impact electron and ion motion. We adapt common electrical and electrochemical techniques, such as four-point probe and Hall Effect measurements, electrochemical impedance spectroscopy, and half-cell electrochemical measurements, to nanomaterial systems, and extend them with capabilities such as air-free measurements or in situ measurements while varying temperature or atmosphere composition [1].
Our effort in the area of ionic charge transport is in in nanoporous CeO2 nanocrystal thin films, using AC impedance spectroscopy in a controlled temperature and gas environment. Here, we found that proton conduction in nanocrystalline metal oxides arises from interface-mediated transport at active surface defect sites, providing controlled doping may enable systematic ionic conductivity tuining in nanocrystal-based composites [1].
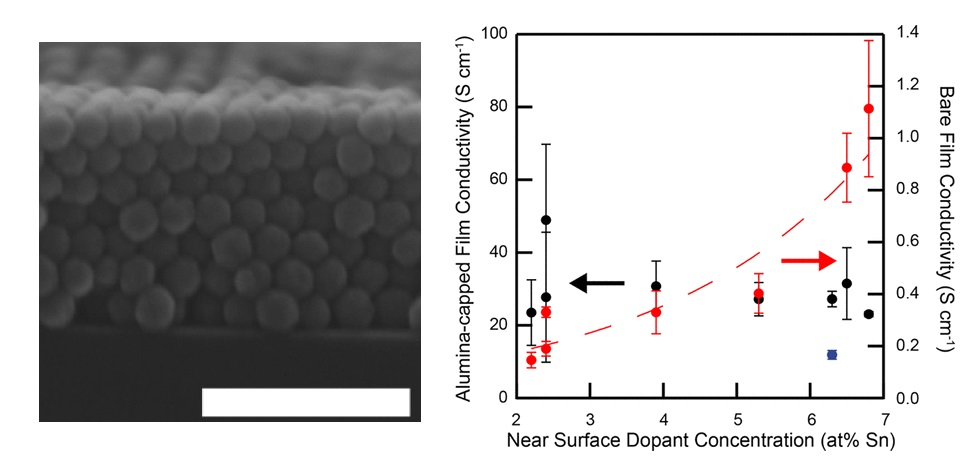
(Left) Electron charge conductive ITO nanocrystal film SEM cross-section. Scale bar at 100 nm. (Right) Electron conductivity of film is shown to be dependent on near-surface dopant concentration [5].
Our research thrust in electronic charge transport include investigating influence of electronic transport by manipulating nanocrystal surface composition and chemistry in nanocrystal films [2,3], fabricating high mobility transparent conductive films by dopant selection [4], and the role of surface vs. homogeneous tin doping in doped indium oxide nanocrystals. In the case of indium tin oxide (ITO) nanocrystals, we have found that surface doping increases the conductivity of nanocrystal films by orders of magnitude by decreasing the nanocrystal's surface depletion width [5]. In Nb-TiO2 nanocrystal films, dynamic charge modulation through capacitive charging was demonstrated with insertion of lithium ions, influencing strong changes in film optical transmittance [6].
Related papers:
[1] EL Runnerstrom, GK Ong, G Gregori, J Maier, and DJ Milliron. “Colloidal Nanocrystal Films Reveal the Mechanism for Intermediate Temperature Proton Conductivity in Porous Ceramics,” J. Phys. Chem. C, 122 (2018), 13624–13635 [link]
[2] J Ephraim, D Lanigan, C Staller, DJ Milliron, E Thimsen. “Transparent Conductive Oxide Nanocrystals Coated with Insulators by Atomic Layer Deposition,” Chem. Mater., 28, (2016), 5549–5553 [link]
[3] E Rosen , AM Sawvel , DJ Milliron , and BA Helms. “Influence of Surface Composition on Electronic Transport Through Naked Nanocrystal Networks,” Chem. Mater. 26, (2014), 2214-2217 [link]
[4] BH Kim, CM Staller, SH Cho, S Heo, CE Garrison, J Kim, and DJ Milliron. “High Mobility in Nanocrystal-Based Transparent Conducting Oxide Thin Films,” ACS Nano, 12 (2018), 3200–3208 [link]
[5] CM Staller, ZL Robinson, A Agrawal, SL Gibbs, BL Greenberg, SD Lounis, UR Kortshagen, and DJ Milliron. “Tuning Nanocrystal Surface Depletion by Controlling Dopant Distribution as a Route Toward Enhanced Film Conductivity,” Nano Lett., 18, (2018), 2870–2878 [link]
[6] Clayton J. Dahlman, Yizheng Tan, Matthew A. Marcus, and Delia J. Milliron. “Spectroelectrochemical Signatures of Capacitive Charging and Ion Insertion in Doped Anatase Titania Nanocrystals,” J. Am. Chem. Soc., 137, (2015), 9160−9166 [link]